Prof. Boris Rubinsky project
in News
I was very glad to be called to produce the Portuguese version of one of Prof. Boris Rubinsky projects (from the University of California, Berkeley). Prof. Boris is one of my academic inspirations. The project is called ‘How to produce hypochlorous acid, the chemical the body uses to fight infections, at home’. This project idea is to provide a way to produce hypochlorous acid at home with very simple materials and proceedings. In addition, to introduce science.
Versions of this video in different languages (as Portuguese, Spanish and Chinese) give to less privileged and non-native English speakers this alternative of hygiene possibility. Fortunately, in Brazil we can produce this type of solution by using bleach, which is easily found in stores at a very low cost! P.S. Besides Brazil and Portugal, Mozambique and Angola also speak Portuguese!
Hypochlorous acid is a weak acid made by the body T cells to fight infections. In electrolysis, it is formed when chlorine dissolves in water and produces powerful oxidizers based on chlorine. Various species are produced in a saline solution as a function of pH. The optimal pH at which all chlorine is hypochlorous acid is produced is about pH 5 as Figure 1 (P.S. This is why vinegar was added in the video!). Hypochlorous acid is recognized by the FDA for sterilization. Click here to see the FDA document. Hypochlorous acid is mild and safe, however - WARNING - the medical grade versions are made in controlled approved medical facilities. Use this home made composition only to disinfect surfaces. Also, click here to see the Science Direct hypochlorous acid page.
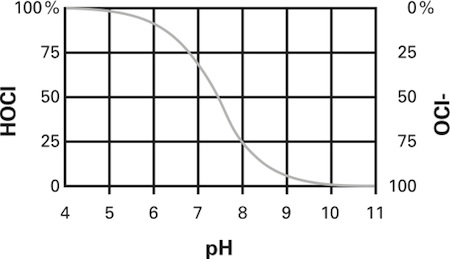
Figure 1 - Hypochlorous acid (HOCl) and hypochlorite ion (OCl-) exist in an equilibrium which is pH dependent. Figure from Cleanroomtechnology.
Check the Portuguese version here:
If you prefer, you can make the solution using bleach dilution. See the video below from the Brazilian Federal Council of Chemistry (in Portuguese):

